


Understanding Hypothyroidism
The Underactive Thyroid
The condition of an underactive thyroid gland, also known as Hypothyroidism, affects nearly five out of one hundred Americans. The average thyroid gland releases T3 and T4 hormones that regulate the body’s metabolic rate, growth, and development. Hypothyroidism occurs when the body does not produce enough thyroid hormones to satisfy bodily functions.

Candidates for T3 Supplementation
While T4 converts to T3 in various parts of the body like the kidneys and liver, some individuals may have impaired conversion processes due to factors like; stress, aging, illness, or genetic variation, which lead to insufficient conversions. Inadequate conversions may cause persistent symptoms for a patient despite having normal T4 levels. In these cases, a synthetic T3 supplementation via liothyronine sodium can help.
Listening to our Community
Through our meetings with endocrinologists and advocacy groups as well as addressing patient inquiries, our company deeply admires the impactful outreach efforts of the thyroid community. At Sigmapharm Laboratories, delivering a stable, reliable, and consistent product for hypothyroid patients has been of upmost importance to us. The idea of LIOMNY™ was born through a clear present demand, and we utilized our innovative technology to produce it.
About LIOMNY™
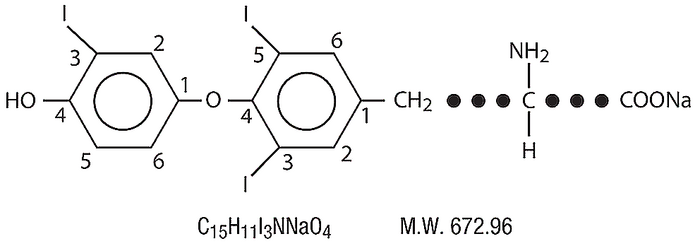
What is Liothyronine Sodium?
Liothyronine sodium, as a synthetic T3 hormone, is particularly beneficial for those who have difficulty converting Thyroxine (T4) to Triiodothyronine (T3). Liothyronine sodium actively binds to thyroid receptors quickly, and effectively reduces symptoms associated with hypothyroidism.

Why LIOMNY™?
LIOMNY™ is the newly branded version of Sigmapharm Laboratories’ Liothyronine Sodium Tablets, USP, and is produced with a strong emphasis on stability, assay, and content uniformity.
LIOMNY™ has a shelf life of five years, making it a highly stable medication with a long-lasting potency. The tablets are produced under strict self-imposed manufacturing specifications for assay and uniformity, which may contribute to consistent therapeutic outcomes for patients.
Learn the Terms

Assay
A measurement to describe the amount of active ingredient present in a pharmaceutical dosage form.
Uniformity
A measurement describing the homogeneity of active ingredient dispersed consistently within a drug product.

Stability
A drug’s ability to remain effective and potent for a specific period of time.
The Importance
During Sigmapharm’s blending, compression, and post packaging process, LIOMNY™ is tested for its self-imposed distribution standard of liothyronine sodium in all batches. Sigmapharm anticipates consistent therapeutic outcomes and is tightening the assay specifications from the USP/NF general standards as indicated below:
USP/NF Assay Acceptance Criteria:
90.0% - 110.0%
LIOMNY™ Assay Acceptance Criteria:
95.0% - 105.0%
The FDA defines liothyronine sodium as a narrow therapeutic index (NTI) drug, meaning that both over-treatment and under-treatment of this medicine can lead to negative effects. To demonstrate product uniformity, and consistency, Sigmapharm Laboratories has tightened the compendial content uniformity requirements by ten percent for LIOMNY™ tablets.
Compendial Uniformity Acceptance Range Tightened by 10.0%
What does this mean for the patient?
Tight assay and uniformity manufacturing specifications in a drug may lead to more accurate reflections of its true dosage strength displayed on the bottle/label. A higher rate of such consistent dosages per tablet may reduce the risk of variability in treatment efficacy.

LIOMNY™ self-imposed manufacturing specifications may affect patient adherence and persistence through consistent therapeutic outcomes and potentially reduced treatment variation.

5 mcg Tablets

25 mcg Tablets

50 mcg Tablets

Reverse Side of all Tablets
All images shown are for illustrative purposes only. Actual product may vary due to image enhancement. The images and information for a particular product may change over time. For the most up-to-date product information, please contact Sigmapharm Laboratories, LLC.
Order LIOMNY™
LIOMNY™ is available for purchase online or over the phone through Marley Drug® with a valid prescription.
Order LIOMNY™ Over the Phone for Mail Order Delivery
Call the Marley Drug Pharmacy directly to order a new prescription or to transfer an existing prescription on your behalf.
1-800-810-7790Order LIOMNY™ Online for Mail Order Delivery
Step 1: Click on the boxed link to redirect you to Marley Drug®.
Step 2: If this is your first-time ordering through Marley Drug®, create an account by selecting “Sign Up” at the top right corner of their page.
Step 3: To begin your purchase, click “Order LIOMNY™ Online” and select your appropriate prescribed strength of 5mcg, 25mcg or 50mcg LIOMNY™(Liothyronine Sodium Tablets, USP) Tablets.
Step 4: Once you have added the order to your cart and selected “Checkout”, continue to the next page and fill out your personal information, prescriber information, delivery address, and payment details.
Step 5: After reviewing your information, submit your order. You will receive an email confirmation as well as a reference code for your purchase. Delivery time is expected to be 2-5 business days.
For inquiries related to LIOMNY™ (Liothyronine Sodium Tablets, USP) please Click here.
For a quick overview, see our flyer
Dosing and Administration
Administer LIOMNY™ orally once daily and individual dosage according to patient response and laboratory findings (See full prescribing information for recommended dosage for hypothyroidism.) TSH suppression in well-differentiated thyroid cancer and for thyroid suppression test. When switching a patient to LIOMNY™ discontinue levothyroxine therapy and initiate LIOMNY™ at a low dosage. Gradually increase the dose according to the patient's response. Adequacy of therapy determined with periodic monitoring of TSH and T3 levels as well as clinical status.
Adults
The recommended starting dosage is 25 mcg orally once daily. Increase the dose by 25 mcg daily every 1 or 2 weeks, if needed. The usual maintenance dose is 25 mcg to 75 mcg once daily. For elderly patients or patients with underlying cardiac disease, start with LIOMNY™ 5 mcg once daily and increase by 5 mcg increments at the recommended intervals. Serum TSH is not a reliable measure of LIOMNY™ dose adequacy in patients with secondary or tertiary hypothyroidism and should not be used to monitor therapy. Use the serum T3 level to monitor adequacy of therapy in this patient population.
In adult patients with primary hypothyroidism, monitor serum TSH periodically after initiation of the therapy or any change in dose. To check the immediate response to therapy before the TSH has had a chance to respond or if your patient's status needs to be assessed prior to that point, measurement of total T3 would be most appropriate. In patients on a stable and appropriate replacement dose, evaluate clinical and biochemical response every 6 to 12 months and whenever there is a change in the patient's clinical status.
Pregnancy
Pre-existing Hypothyroidism: Thyroid hormone dose requirements may increase during pregnancy. Measure serum TSH and free-T4 as soon as pregnancy is confirmed and, at minimum, during each trimester of pregnancy. In patients with primary hypothyroidism, maintain serum TSH in the trimester-specific reference range. For patients with serum TSH above the normal trimester-specific range, increase the dose of thyroid hormone and measure TSH every 4 weeks until a stable dose is reached and serum TSH is within the normal trimester-specific range. Reduce thyroid hormone dosage to pre-pregnancy levels immediately after delivery and measure serum TSH levels 4 to 8 weeks postpartum to ensure thyroid hormone dose is appropriate.
INDICATIONS FOR LIOMNY™
INDICATIONS AND USAGE
LIOMNY™ is an L-triiodothyronine (T3) indicated for:
Hypothyroidism
As replacement in primary (thyroidal), secondary (pituitary), and tertiary (hypothalamic) congenital or acquired hypothyroidism.
Pituitary Thyrotropin (Thyroid-Stimulating Hormone, TSH) Suppression
As an adjunct to surgery and radioiodine therapy in the management of well-differentiated thyroid cancer.
Thyroid Suppression Test
As a diagnostic agent in suppression tests to differentiate suspected mild hyperthyroidism or thyroid gland autonomy.
Limitations of Use:
LIOMNY™ is not indicated for suppression of benign thyroid nodules and nontoxic diffuse goiter in iodine-sufficient patients. LIOMNY™ is not indicated for treatment of hypothyroidism during the recovery phase of subacute thyroiditis.
IMPORTANT SAFETY INFORMATION
WARNING: NOT FOR TREATMENT OF OBESITY OR FOR WEIGHT LOSS
Thyroid hormones, including LIOMNY™, either alone or with other therapeutic agents, should not be used for the treatment of obesity or for weight loss. In euthyroid patients, doses within the range of daily hormonal requirements are ineffective for weight reduction. Larger doses may produce serious or even life-threatening manifestations of toxicity, particularly when given in association with sympathomimetic amines such as those used for their anorectic effects.
Dosing and Administration
Administer LIOMNY™ orally once daily and individual dosage according to patient response and laboratory findings. (See full prescribing information for recommended dosage for hypothyroidism.) TSH suppression in well-differentiated thyroid cancer and for thyroid suppression test. When switching a patient to LIOMNY™ discontinue levothyroxine therapy and initiate LIOMNY™ at a low dosage. Gradually increase the dose according to the patient's response. Adequacy of therapy determined with periodic monitoring of TSH and T3 levels as well as clinical status.
Dosage Forms And Strengths
Tablets: 5 mcg, 25 mcg, 50 mcg.
CONTRAINDICATIONS
LIOMNY™ is contraindicated in patients with uncorrected adrenal insufficiency.
Warnings and Precautions
Cardiac adverse reactions in the elderly and in patients with underlying cardiovascular disease: Initiate LIOMNY™ at less than the full replacement dose because of the increased risk of cardiac adverse reactions, including atrial fibrillation.
Myxedema coma: Do not use oral thyroid hormone drug products to treat myxedema coma.
Acute adrenal crisis in patients with concomitant adrenal insufficiency: Treat with replacement glucocorticoids prior to initiation of LIOMNY™ treatment.
Prevention of hyperthyroidism or incomplete treatment of hypothyroidism: Proper dose titration and careful monitoring is critical to prevent the persistence of hypothyroidism or the development of hyperthyroidism.
Worsening of diabetic control: Therapy in patients with diabetes mellitus may worsen glycemic control and result in increased antidiabetic agent or insulin requirements. Carefully monitor glycemic control after starting, changing, or discontinuing thyroid hormone therapy.
Decreased bone mineral density associated with thyroid hormone over-replacement: Over-replacement can increase bone resorption and decrease bone mineral density. Give the lowest effective dose.
Adverse Reactions
Most common adverse reactions for LIOMNY™ are primarily those of hyperthyroidism due to therapeutic overdosage: arrhythmias, myocardial infarction, dyspnea, headache, nervousness, irritability, insomnia, tremors, muscle weakness, increased appetite, weight loss, diarrhea, heat intolerance, menstrual irregularities, and skin rash.
To report SUSPECTED ADVERSE REACTIONS, contact Sigmapharm Laboratories, LLC,
Pharmacovigilance at 1-855-332-0731 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See full prescribing information for drugs that affect thyroid hormone pharmacokinetics and metabolism (e.g., absorption, synthesis, secretion, catabolism, protein binding, and target tissue response) and may alter the therapeutic response to LIOMNY™.



